Background:
Patients (pts) with relapsed/refractory (R/R) DLBCL and iNHL who are ineligible for, or relapse after, high-dose chemotherapy (HDC)/autologous stem cell transplantation (ASCT) have a poor prognosis and limited treatment options. Selinexor, a selective inhibitor of nuclear export has received accelerated approval by the U.S. Food and Drug Administration, for the treatment of adult patients with R/R DLBCL. Phase I/II study (NCT05265975), selinexor in combination with lenalidomide and rituximab (R2) for R/R DLBCL and iNHL, is ongoing. Phase I results were previously reported at ICML 2023 (Weili Zhao et al., ICML 2023, #658) and the recommended phase 2 dosing (RP2D) was determined.
Aims:
Here we update the preliminary results of SWATCH study.
Methods:
Additional 6 subjects will be recruited at this dose level 60mg to further confirm the safety and tolerability of RP2D. Eligible pts were treated with 6 cycles of rituximab (375mg/m2 on day 1), lenalidomide (25mg on d1-10) and selinexor (dose level: 60mg, on days 1, 8,15 of each 28-day cycle), followed by selinexor and lenalidomide maintenance until disease progression. Dose limiting toxicities (DLT) was defined as the occurrence of severe toxicities during the first cycle: grade 3 febrile neutropenia> 5 days, grade 4 neutropenia or thrombocytopenia >7 days, grade 3/4 thrombocytopenia with hemorrhage, or any grade 3 non-hematologic toxicity >7 days.
Results:
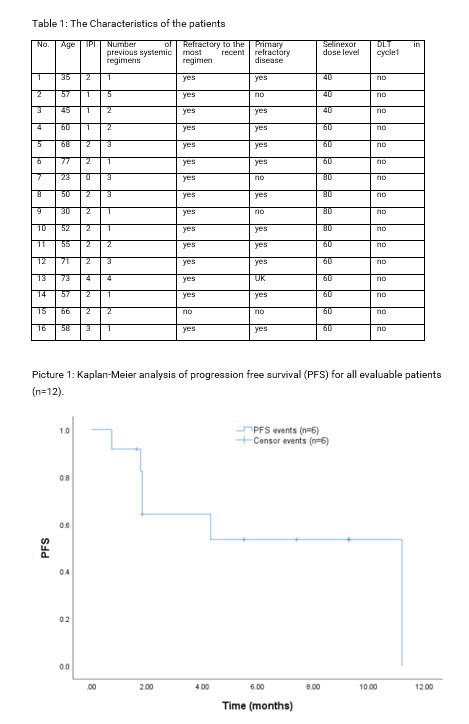
From May 2022 to April 2023, 16 pts were enrolled and no DLT occurred. At baseline in these 16 pts, refractory to last line was 93.75% and primary refractory was 68.75%. Among 12 efficacy evaluable pts, 3 pts achieved complete response (CR), 5 pts achieved partial response (PR) and another 4 pts achieved progressive disease (PD). Most AEs were grade 1 or 2 and were reversible with supportive care or dose modification. At data cutoff (June 1, 2023), 7 pts were still receiving treatment and no pts came off study due to intolerability or AEs. Median PFS and median DOR were 11.2 months and not reached.
Conclusion:
Selinexor in combination with lenalidomide and rituximab showed encouraging preliminary efficacy and generally tolerable toxicity with an ORR of 66.7%, CR of 25% and median PFS 11.2 months in all evaluable dose level pts, and with an ORR of 80% and CR of 20% in evaluable selinexor 60mg dose (RP2D). This study is currently enrolling patients in the dose expansion group.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Selinexor, a selective inhibitor of nuclear export has been approved by the US Food and Drug Administration for the treatment of R/R DLBCL. We want to ehvaluating the safety and tolerability of selinexor in combination with R2 for R/R DLBCL and iNHL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal